Society is rapidly shifting away from fossil fuels to renewable resources and electric batteries. Despite the pressing need to transition to greener techniques, core challenges concerning efficiency and sustainability remain. For example, the sluggish charging speeds of lithium-ion (Li-ion) batteries used in electric vehicles are impeding widespread market adoption.
 In an effort to improve performance in lithium-ion batteries, a group of researchers from Japan Advanced Institute of Science and Technology synthesized a lithium borate-type aqueous polyelectrolyte binder for graphite anodes. Their new binder helped improve Li-ion diffusion and lower impedance compared to conventional batteries. Image Credit: Noriyoshi Matsumi from JAIST
In an effort to improve performance in lithium-ion batteries, a group of researchers from Japan Advanced Institute of Science and Technology synthesized a lithium borate-type aqueous polyelectrolyte binder for graphite anodes. Their new binder helped improve Li-ion diffusion and lower impedance compared to conventional batteries. Image Credit: Noriyoshi Matsumi from JAIST
The “holy grail” of features aspired by the automobile sector in batteries include “extreme” quick charging (where 80% of the battery is charged in 10 minutes), high energy density, and cycle life.
To allow fast-charging capability in batteries, researchers have long worked to improve electrolyte mass transfer and charge transfer in electrodes, with more emphasis placed on the former than the latter.
A recent study by Professor Noriyoshi Matsumi of Japan Advanced Institute of Science and Technology (JAIST) demonstrates a new way to facilitate quick charging by employing a binder material that facilitates Li-ion intercalation of active material.
The binder material improves desolvated Li ion transport across the solid electrolyte interface (SEI) and within the anode material, resulting in high conductivity, low impedance, and good stability.
Former senior lecturer Rajashekar Badam, postdoctoral research fellow Anusha Pradhan, former graduate student Ryoya Miyairi, and doctoral course student Noriyuki Takamori from JAIST were part of the team. Their study was published in the journal ACS Materials Letters.
Study corresponding authors and professors Matsumi and Badam of JAIST stated, “Our current strategy of using a bio-derived lithium borate polymer as aqueous polyelectrolyte binder to enhance charge transfer within electrodes such as graphite anodes exhibits fast charging capability.”
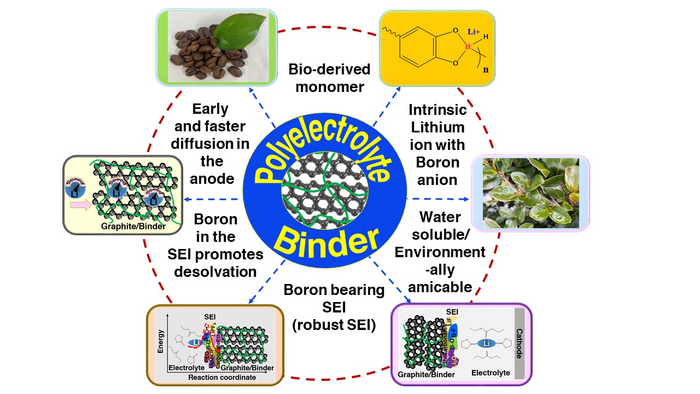
While most battery research focuses on the design of active materials and enhanced electrolyte mass transfer, the current study takes a different approach by designing a special binder material that facilitates lithium-ion intercalation of the active material.
The binder material includes highly dissociable lithium borate, which improves lithium-ion diffusion in the anode matrices. Further, this binder can form an organoboron SEI, which shows very low interfacial resistance when compared with ordinary battery cells.
Noriyoshi Matsumi, Study Corresponding Author and Professor, Japan Advanced Institute of Science and Technology
Boron compounds (such as tetracordinate boron in the binder and boron-rich SEI) help in the desolvation of Li+ ions by lowering the activation energy of Li+ desolvation from the solvent sheath at the SEI. Also, the overpotential associated with charge transfer at the interface is decreased with high diffusion and low impedance.
This is one of the important determining factors for extreme fast charging.
Anusha Pradhan, Study First Author and Postdoctoral Research Fellow, Japan Advanced Institute of Science and Technology
Li plating occurs on graphite electrodes when charging exceeds the rate of intercalation. It is an unwanted procedure that reduces battery life and limits quick charging capability. In the current study, enhanced ion transport over the SEI and inside the electrodes reduces the concentration polarization of Li+ ions, resulting in the lack of graphite plating.
The researchers not only provided a unique technique for extremely high-rate charging batteries with reduced interfacial resistance in their study but also employed a biopolymer produced from caffeic acid.
Caffeic acid, a plant-based organic compound, is a sustainable and ecologically safe source of the material. As a result, although the market for these batteries expands rapidly, the use of bio-based resources in these batteries reduces carbon dioxide emissions.
Underlining the essential capabilities of the structure employed in this study, Prof. Matsumi added, “In future studies, our binder can also be combined with high-rate chargeable active materials to enable further synergistic effect in enhancing performance.”
With increasing studies into battery performance, it is reasonable to expect greener solutions in the way energy is utilized, particularly in the transportation sector, in the near future.
Prof. Matsumi concluded, “Through the high-rate chargeable battery technology, people will enjoy electric vehicles and convenient mobile devices. As the use of renewable resources will maintain availability of products for long, irrespective of availability of fossil resources and influences by high social situations.”
Journal Reference:
Pradhan, A., et al. (2023) Extreme Fast Charging Capability in Graphite Anode via a Lithium Borate Type Biobased Polymer as Aqueous Polyelectrolyte Binder ACS Materials Letters. doi:10.1021/acsmaterialslett.2c00999.