Gold (Au)-based electrocatalysts used in water electrolysis for hydrogen production display high chemical stability but low hydrogen evolution reaction (HER) activity. Nickel (Ni) alloying can enhance their HER activity.
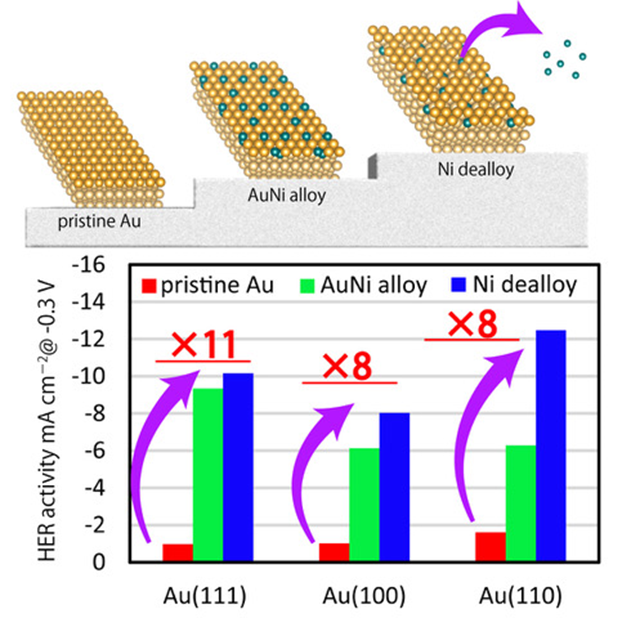
AuNi surface alloy prepared on single-crystal Au electrode in sulfuric acid. Hydrogen evolution reaction activity of AuNi/Au catalysts is enhanced by Ni dealloying and depends on the surface structure of the Au substrate, with (110) surface resulting in the highest activity followed by (111) and (100), respectively. Image Credit: Masashi Nakamura from Chiba University
Chiba University researchers recently evaluated the HER activity and surface properties of AuNi alloy prepared on single crystal Au surfaces, disclosing the atomic structural changes and surface sites responsible for the increased HER activity of the AuNi/Au catalyst during electrolysis.
Hydrogen gas has gained popularity as the fuel for a clean and sustainable future in recent years. Water vapor is produced as a byproduct of the combustion of this carbon-neutral fuel source, which releases enormous amounts of energy. Electricity is used to split water into hydrogen and oxygen, which is one of the most common ways to produce hydrogen.
Water is split using an electrochemical cell, and during the hydrogen evolution reaction (HER), the hydrogen gas is released at the negatively charged electrode.
Catalysts are used to reduce the HER overpotential (the difference between the theoretical cell voltage and the voltage required for carrying out hydrogen evolution) to increase the efficiency of the process.
Recently, a gold (Au) and nickel (Ni) alloy demonstrated promising HER activity. While the electrochemical properties of AuNi have been extensively studied, little is known about its surface structure and atomic composition, which govern a catalyst's electrocatalytic activity.
Chiba University researchers, guided by Associate Professor Masashi Nakamura of the Graduate School of Engineering and including doctoral student Syunnosuke Tanaka of the Graduate School of Science and Engineering and Professor Nagahiro Hoshi of the Graduate School of Engineering, have now bridged the gap in understanding of AuNi electrocatalysts.
The group analyzed the surface structure, atomic arrangement, and HER activity of AuNi surface alloys prepared at different alloying temperatures on single-crystal Au electrodes in the article published in ChemElectroChem on June 28th, 2023.
Rare and highly expensive metals like platinum are commonly used as catalysts for water electrolysis. While Au shows high chemical stability as a catalyst compared to platinum, it suffers from low HER activity. Now, AuNi nanoparticles have emerged as a promising non-platinum alternative, and it is crucial to improve their HER activity further.
Masashi Nakamura, Associate Professor, Graduate School of Engineering, Chiba University
The researchers placed the AuNi/Au electrode in an electrochemical cell containing 0.05 M sulfuric acid and measured its HER activity using cyclic voltammograms (CV) and linear sweep voltammograms (LSV). X-Ray photoelectron spectroscopy (XPS) and surface X-Ray diffraction (SXRD) techniques were also used to examine the surface properties of the AuNi/Au catalyst.
The HER activity of AuNi/Au was found to be dependent on the surface structure of the Au substrate, with (110) surface resulting in the highest activity, followed by (111) and (100), respectively. Furthermore, the surface alloy increased HER activity via Ni dealloying.
This was confirmed by XPS and SXRD, which revealed a decrease in atomic occupancy on the topmost layer of the surface due to Ni dissolution from the surface-alloy layer. The surface defects caused by the Ni dealloying process activated the HER, as did the low-coordination Au sites next to Ni.
The current research sheds light on the structural and electrochemical properties of AuNi surface alloys, laying the groundwork for highly active and long-lasting Au-based catalysts for practical electrolysis and fuel cell applications.
Designing effective non-platinum electrocatalysts can reduce the cost of water electrolysis and also improve its energy conversion efficiency, which is crucial for accelerating toward a hydrogen-driven society.
Masashi Nakamura, Associate Professor, Graduate School of Engineering, Chiba University
Journal Reference
Tanaka, S., et al. (2023). Hydrogen Evolution Reaction on AuNi Surface Alloy Formed on Single Crystal Au Electrodes. ChemElectroChem. doi.org/10.1002/celc.202300095.