Incorporating charged lithium ions within polyoxometalates (POMs), when combined with ionic liquids, enhances the ion conductivity of a solid-state electrolyte membrane.
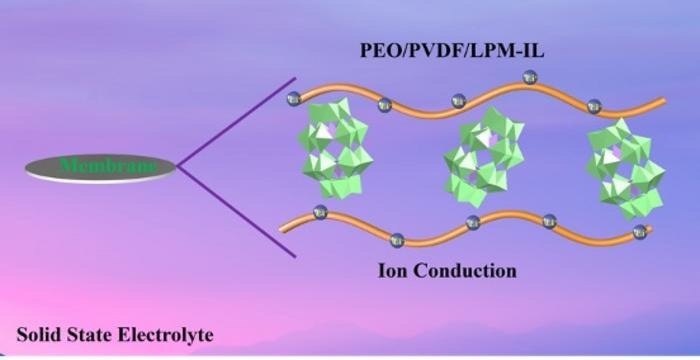 The green polyoxometalate-based lithium salt (LPM) is sandwiched between two layers of an ionic liquid (orange) that promotes the dissociation of lithium ions from LPM, increasing the ion conductivity of the composite solid electrolyte membrane. Image Credit: Polyoxometalates, Tsinghua University Press.
The green polyoxometalate-based lithium salt (LPM) is sandwiched between two layers of an ionic liquid (orange) that promotes the dissociation of lithium ions from LPM, increasing the ion conductivity of the composite solid electrolyte membrane. Image Credit: Polyoxometalates, Tsinghua University Press.
Solid-state lithium-ion batteries operate by facilitating the movement of ions (charged atoms) within a solid medium, as opposed to a liquid state, for both charging and discharging. These solid-state electrolytes offer enhanced safety, cost-efficiency, and the potential for higher energy densities compared to batteries reliant on liquid electrolytes.
Nevertheless, they face challenges such as low ionic conductivity, which hampers ion movement, and limited thermal stability. To address these issues, a novel composite solid electrolyte (CSE) membrane was created by combining lithium salts and an ionic liquid. This development aims to improve the dissociation of charged lithium ions within a solid-state electrolyte battery, consequently enhancing its conductivity.
Polyoxometalates (POMs) consist of clusters of metal and oxygen atoms, and their properties are governed by the well-defined structure of these atom clusters.
Researchers from Northeast Normal University recently introduced a POM-based lithium salt, Li6P2Mo18O62 (LPM), into a solid-polymer electrolyte (SPE) composed of the polymer polyethylene oxide (PEO), a cost-effective and stable polymer comprised of numerous ethylene oxide subunits.
PEO is known for its limited ionic conductivity, but the addition of LPM salt modifies the polymer's characteristics and enhances ion mobility. To further improve the conductivity of the composite electrolyte material, the research team also integrated an ionic liquid (IL) to liberate lithium ions from LPM.
The research was published on September 28, 2023, in the journal Polyoxometalates.
Solid-state electrolytes (SSEs) are considered… the most promising candidates for next-generation energy storage devices due to their excellent thermal and electrochemical stability. Although SPEs have excellent flexibility and viscosity, they are severely limited due to their low ionic conductivity, poor mechanical strength, and low thermal stability at room temperature. In contrast, inorganic solid electrolytes (ISEs) like LPM... usually have high ionic conductivity.
Hong-Ying Zang, Study Senior Author and Professor, Key Laboratory of Polyoxometalate and Reticular Material Chemistry, Northeast Normal University
Hong-Ying Zang adds, “By incorporating ISEs like LPM into SPEs to form composite polymer electrolytes, we leverage their respective properties… to achieve optimized mechanical properties and improve their ionic conductivity.”
Currently, inorganic electrolyte fillers include nanoparticles… and ionic-conductive inorganics. As a class of metal-oxygen clusters, the application of polyoxometalates in solid-state batteries is hampered by the difficulty of moving lithium ions. In this paper, we promote the dissociation of lithium ions from polyoxometalates with ILs… to impart LPM and IL composites (LPM-IL) with good electrical conductivity.
Hong-Ying Zang, Study Senior Author and Professor, Key Laboratory of Polyoxometalate and Reticular Material Chemistry, Northeast Normal University
The research team evaluated the ion conductivity and mobility of the composite membrane by gauging AC impedance, which measures the resistance to current flow in a circuit. They observed that electrolyte membranes containing the ideal concentration of LPM and IL exhibited conductivity three times greater than membranes prepared without IL.
Likewise, the team established that the conductivity of composite membranes produced using polyvinylidene fluoride (PVDF), a non-reactive thermoplastic filler material, alongside PEO, exhibited a tenfold increase in conductivity when compared to LPM-IL membranes crafted without PVDF. Furthermore, the composite membrane displayed commendable stability, maintaining its performance for a period of 12 hours at a temperature of 80 ℃.
“The results of these experiments demonstrate that polyoxometalates can be used as inorganic solid electrolytes,” notes Zang.
IL effectively enhanced the separation of lithium ions from LPM, consequently improving the ionic conductivity of the composite solid electrolyte membrane. Furthermore, the integration of PVDF resulted in the formation of a conductive PEO-PVDF network within the membrane, which further facilitated the movement of lithium ions and enhanced overall conductivity.
The research team believes their unique, PEO-based composite membrane containing PVDF, POM-based lithium salt, and IL provides a practical means of increasing ionic conductivity in solid-state electrolytes for use in lithium-ion batteries.
Our next step is to improve the performance of polyoxometalates to create better solid-state lithium-ion batteries.
Hong-Ying Zang, Study Senior Author and Professor, Key Laboratory of Polyoxometalate and Reticular Material Chemistry, Northeast Normal University
Qianqian Liu, Yunzuo Cui, Lijie Zhu, Dongming Cheng, Chen Wang, Siqi Lu, Bo Li, and Xinyu Chen from the Key Laboratory of Polyoxometalate and Reticular Material Chemistry at the Ministry of Education at Northeast Normal University in Changchun, China contributed to the study.
The research was funded by the National Natural Science Foundation of China (Grant No. 22302102, 21871042, 21471028, 22073094), the Fundamental Research Funds for the Central Universities-Excellent Youth Team Program (2412023YQ001), the Natural Science Foundation of Jilin Province (Grant No. 20200201083JC) and the Natural Science Foundation of Department of education of Jilin Province (JJKH20201169KJ).
Journal Reference:
Liu, Q., et al. (2023). Ionic liquid-mediated PEO-based solid-state electrolyte membrane modified with Dawson-type polyoxometalates. Polyoxometalates. doi.org/10.26599/pom.2023.9140036.