In a notable advancement for chemistry, innovators at the Department of Energy's Oak Ridge National Laboratory have devised a closed-loop process for synthesizing an exceptionally resilient carbon-fiber-reinforced polymer (CFRP) and subsequently recovering all of its initial components.
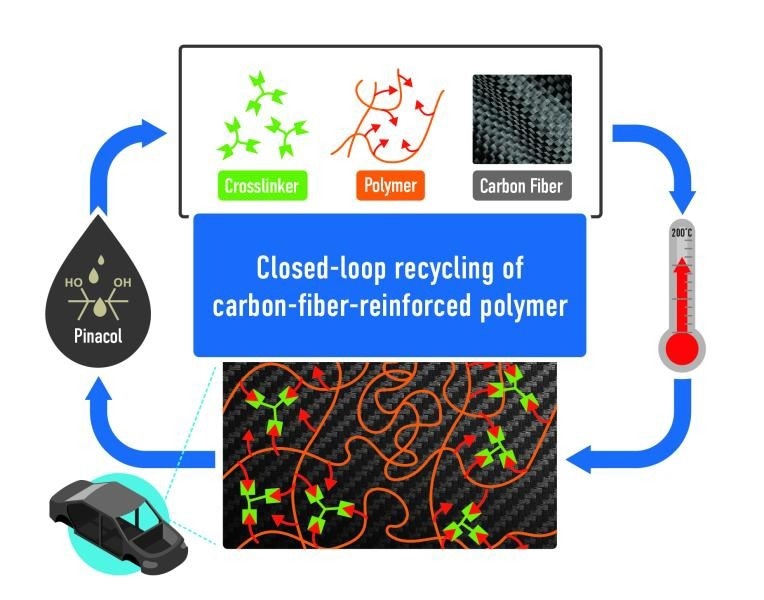 A polymer, functionalized carbon fibers, and a crosslinker are mixed and cured. The components can be retrieved by the addition of an alcohol, pinacol. Image Credit: Philip Gray and Anisur Rahman/ORNL, US Dept. of Energy
A polymer, functionalized carbon fibers, and a crosslinker are mixed and cured. The components can be retrieved by the addition of an alcohol, pinacol. Image Credit: Philip Gray and Anisur Rahman/ORNL, US Dept. of Energy
CFRP, a composite material known for its lightweight, strength, and durability, plays a crucial role in reducing weight and enhancing the fuel efficiency of vehicles such as automobiles, airplanes, and spacecraft.
However, traditional CFRPs pose challenges in terms of recycling, often ending up as single-use materials with significant carbon footprints. In contrast, ORNL's closed-loop technology, detailed in a publication in Cell Reports Physical Science, offers a promising solution to this formidable challenge.
We incorporated dynamic crosslinking into a commodity polymer to functionalize it. Then, we added a crosslinker to make it like thermoset materials. Dynamic crosslinking allows us to break chemical bonds and reprocess or recycle the carbon fiber composite materials.
Md Anisur Rahman, Chemist and Inventor, Oak Ridge National Laboratory
A traditional thermoset material is permanently crosslinked, meaning it cannot be reprocessed once it is synthesized, cured, molded, and shaped. However, ORNL's system introduces dynamic chemical groups to the polymer matrix and its embedded carbon fibers.
This unique approach allows the polymer matrix and carbon fibers to undergo multiple reprocessing cycles without experiencing any degradation in mechanical properties, such as strength and toughness.
Rahman spearheaded the study alongside ORNL Chemist Tomonori Saito, who received recognition from Battelle in 2023 as the ORNL Inventor of the Year.
Most experiments were carried out by Rahman and ORNL postdoctoral fellow Menisha Karunarathna Koralalage. Together, this team has submitted a patent application for their innovative work.
We invented a tough and recyclable carbon fiber composite. The fiber and the polymer have a very strong interfacial adhesion due to the presence of dynamic bonds.
Tomonori Saito, Chemist, Oak Ridge National Laboratory
The interface securely binds materials together through covalent interactions and can be selectively loosened as needed using either heat or specific chemical processes.
Saito added, “The functionalized fiber has dynamic exchangeable crosslinking with this polymer. The composite structure is really tough because of the interface characteristics. That makes a very, very strong material.”
Conventional polymers like thermoset epoxies are commonly employed to permanently bond materials such as metal, carbon, concrete, glass, ceramic, and plastic, forming multicomponent materials like composites.
However, in the ORNL material, once the polymer, carbon fibers, and crosslinker have undergone thermosetting, they can be reverted back into their initial materials. This unique property allows the components of the material to be released for recycling when a specific alcohol, known as pinacol, replaces the covalent bonds of the crosslinker.
Closed-loop recycling at the laboratory scale results in no loss of starting materials. “When we recycle the composites, we recover 100% of the starting materials — the crosslinker, the polymer, the fiber,” Rahman added.
“That’s the importance of our work,” Saito says. “Other composite recycling technologies tend to lose the component starting materials during the recycling process.”
The reversibly crosslinked CFRPs offer several additional advantages, including rapid thermosetting, self-adhesive properties, and the ability to repair microcracks within the composite matrix.
Looking ahead, closed-loop recycling of CFRPs has the potential to revolutionize low-carbon manufacturing by integrating circular lightweight materials into clean-energy technologies.
The researchers drew inspiration from nature, which utilizes dynamic interfaces to produce resilient materials. For instance, nacre, the iridescent mother-of-pearl found inside the shells of marine mussels and other mollusks, exhibits exceptional toughness, capable of deforming without fracturing.
Furthermore, marine mussels demonstrate strong adhesion to surfaces while effectively dissipating energy to detach when needed. Building on these principles, the researchers aimed to optimize the interfacial chemistry between the carbon fibers and the polymer matrix to enhance interfacial adhesion and bolster the toughness of CFRPs.
Our composite’s strength is almost two times higher than a conventional epoxy composite. Other mechanical properties are also very good.
Md Anisur Rahman, Chemist and Inventor, Oak Ridge National Laboratory
The tensile strength, representing the stress a material can withstand when pulled, reached a record high among similar fiber-reinforced composite materials, measuring at 731 megapascals. This surpassed the strength of stainless steel and exceeded that of conventional epoxy-based CFRP composites used in automobiles.
Moreover, in the ORNL material, the dynamic covalent bonding between the fiber interface and the polymer exhibited a 43% increase in interfacial adhesion compared to polymers lacking dynamic bonds.
The presence of dynamic covalent bonds facilitates closed-loop recycling. In conventional matrix materials, separating carbon fibers from the polymer proves challenging. However, ORNL's chemical approach, which cleaves fibers at functional sites, enables the separation of fibers from the polymer for reuse.
Karunarathna Koralalage, Rahman, and Saito, with assistance from Natasha Ghezawi, a Graduate Student at the Bredesen Center for Interdisciplinary Research and Graduate Education of the University of Tennessee, Knoxville, modified a commodity polymer known as S-Bpin.
They developed an upcycled styrene ethylene butylene styrene copolymer, incorporating boronic ester groups that form covalent bonds with a crosslinker and fibers, resulting in the formation of the durable CFRP.
Due to the complexity of CFRP, its comprehensive characterization necessitated a diverse range of expertise and advanced instrumentation. Chris Bowland at ORNL conducted tests on tensile properties, while Guang Yang utilized Raman mapping to illustrate the distribution of chemical and structural species. Catalin Gainaru and Sungjin Kim, both from ORNL, contributed rheological data, which was further analyzed by Alexei Sokolov, a UT-ORNL Governor’s Chair. Bingrui Li, affiliated with ORNL and UT, employed scanning electron microscopy to demonstrate the maintenance of carbon fiber quality after recycling.
Vivek Chawla and Dayakar Penumadu, both from UT, undertook the analysis of interlaminar shear strength. Harry Meyer III at ORNL utilized X-Ray photoelectron spectroscopy to identify molecules attached to fiber surfaces. Furthermore, the paper was reviewed by ORNL's Amit Naskar, a distinguished expert in carbon fiber.
The researchers discovered that the extent of dynamic crosslinking plays a crucial role in the material's performance.
Rahman added, “We found 5% crosslinking works better than 50%. If we increase the crosslinker amount, it starts making the polymer brittle. That’s because our crosslinker has three hand-like bulky structures, able to make more connections and decrease the polymer’s flexibility.”
In their next phase, the research team intends to explore similar studies involving glass-fiber composites. This endeavor aims to uphold high-performance standards while simultaneously reducing costs and carbon footprints across various applications, including aerospace, automotive, marine, sporting goods, construction, and engineering.
Additionally, the team aims to streamline the production costs of the new technology to enhance its commercial viability for potential future licensees.
“This step will open more applications, especially for wind turbines, electric vehicles, aerospace materials, and even sporting goods,” Rahman noted.
The research was sponsored by the Vehicle Technologies Office within the Department of Energy's Office of Energy Efficiency and Renewable Energy. Additionally, Raman mapping was sponsored by DOE's Office of Electricity.
UT-Battelle oversees the management of ORNL on behalf of DOE's Office of Science. The Office of Science, which stands as the largest supporter of basic research in the physical sciences within the United States, is dedicated to tackling some of the most urgent challenges of our era.