Jan 13 2016
Engineers from Northwestern University have created a new technique to print 3D metallic objects without relying on metal powder beds, and expensive electron beams or lasers. This technique is fast, cheap, and provides a more uniform process, utilizing common furnaces and liquid inks. The research team also demonstrate that the new method could be used for a varied range of metals, alloys, metal mixtures, compounds, and metal oxides.
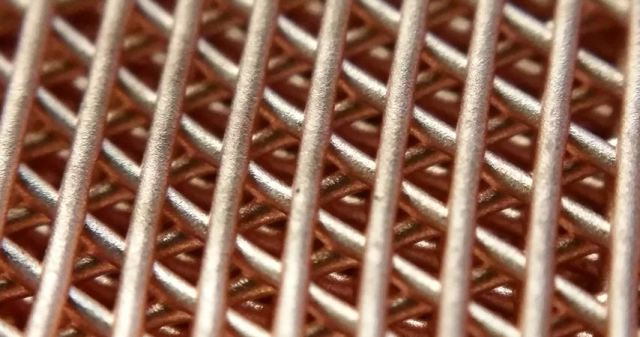 Metals and Alloys" />A copper lattice structure created with Northwestern Engineering’s new 3-D printing process.
Metals and Alloys" />A copper lattice structure created with Northwestern Engineering’s new 3-D printing process.
This is exciting because most advanced manufacturing methods being used for metallic printing are limited as far as which metals and alloys can be printed and what types of architecture can be created. Our method greatly expands the architectures and metals we're able to print, which really opens the door for a lot of different applications.
Ramille Shah, Assistant Professor of Materials Science and Engineering, McCormick School of Engineering
Traditional 3-D printing techniques used for metallic structures are expensive and time consuming. These traditional methods use an intense energy source, like an electron beam or focused laser, that moves across a layer of metal powder and fuses the powder particles together. The steps are repeated when a new powder is placed on top of the previous layer, which eventually creates a 3-D object. Powder that is left unfused is removed, this is to ensure certain architectures aren't created, much like those that are hollow and enclosed. This technique is also restrained by the types of metals and alloys that are compatible.
In their new technique Northwestern Engineers totally ruled out the need for the energy beam and powder approach, they also uncouple the two-step process of printing the structure and fusing its layers. Ramille Shah created a liquid ink made up of an elastomeric binder, solvents, and mixed metal or metal powders. Shah was then able to use a simple syringe-extrusion process to dispense ink through a nozzle and rapidly print structures at room temperature.
Although the process starts with liquid ink, the dispensed material immediately solidifies and fuses with the material that was extruded earlier. This enables very big objects to be created quickly and handled immediately. The structure is then heated in a simple furnace and the powder is combined together without melting. This process of heating is known as sintering and was conducted by the team with collaborator David Dunand, the James N, Margie M. Krebs Professor of Materials Science and Engineering.
By uncoupling the printing and the sintering, it appears that we have complicated the process. But, in fact, it has liberated us as each step is much easier separately than the combined approach.
David Dunand, Professor of Materials Science and Engineering, Northwestern University
This research can be found in the last month’s publication of the Advanced Functional Materials journal. Shannon L. Taylor, a graduate student who is supported by a National Science Foundation graduate fellowship, and Adam Jakus, a postdoctoral fellow in Shah's laboratory who has support from the Department of Defense fellowship, under the guidance of Shah and Dunand were the co-first authors. The paper was also co-authored by Nicholas R. Geisendorfer, an undergraduate.
The research team expects many disciplines to benefit from this method, it could be used to quickly print batteries, solid-oxide fuel cells, medical implant, and mechanical parts for bigger structures, such as rockets and airplanes. This unique 3-D ink and process open doors for more sophisticated and uniform architectures that are faster to create and easier to scale up. Before the object is densified by heating the structure, called a “green body”, is flexible because of the unbonded metallic powders from the elastic polymer binder.
We used a biomedical polymer that is commonly used in clinical products, such as sutures. When we use it as a binder, it makes green bodies that are very robust despite the fact that they still comprise a majority of powder with very little binder. They're foldable, bendable, and can be hundreds of layers thick without crumbling. Other binders don't give those properties to resulting 3-D printed objects. Ours can be manipulated before being fired. It allows us to create a lot of different architectures that haven't really been seen in metal 3-D printing.
Ramille Shah, Assistant Professor of Materials Science and Engineering, McCormick School of Engineering
The green body leads to more uniform structures once they are heated in the furnace and simultaneously densify. In the traditional method the heat is localized and can create heating and cooling stresses, which results in an undesirable microstructure and eventually, suboptimal properties. Usage of a furnace holds an upper hand, as it provides uniform temperature, which allows structures to sinter uniformly, without cracking or warping.
To me, as a metallurgist, I'm amazed that the structure does not deform or break apart, despite shrinking extensively during densification. That is not something that I see often.
David Dunand, Professor of Materials Science and Engineering, Northwestern University
This method, discovered by Shah and Dunand, uses many extrusion nozzles at a single time, instead of a single laser working slowly across a large powder bed. By doing this they can print full sheets of 3D structures that are meters wide, and
these sheets can be folded into large structures. The only constraint in their method is the furnace size.
This method is also capable of printing metal oxides, which can then be reduced into their respective metal. One example of a metal oxide is iron oxide (rust), and using rust powder is advantageous over pure iron powders due to it being cheap, stable, and safe. After printing with rust and other metallic oxides, Shah’s research team used hydrogen to change the green bodies into their respective metals before they were sintered in the furnace.
It might seem like we are needlessly complicating things by adding a third reduction step where we turn rust into iron. But this opens up possibilities for using very cheap oxide powders rather than corresponding expensive metal powders. It's hard to find something cheaper than rust.
David Dunand, Professor of Materials Science and Engineering, Northwestern University