Traditional methods, such as topical or systemic antibiotic administration, may not be effective in preventing colonization, even though it is a major cause of failure and clinical complications for load-bearing metallic orthopedic implants. This is especially true in the case of secondary infection, which frequently necessitates surgical removal of the implants and, in extreme cases, even limb amputation.
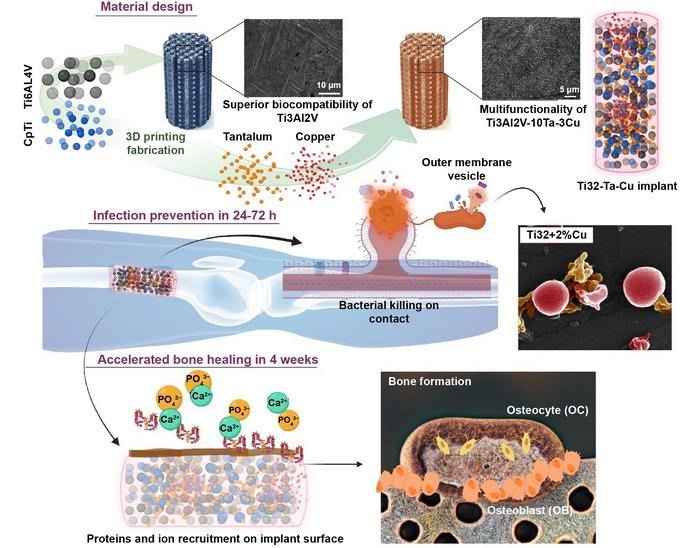 These implants are fabricated via multi-material 3D Printing or additive manufacturing to impart continued bacterial resistance while enabling early bone formation through enhanced biocompatibility. Image Credit: Amit Bandyopadhyay, Indranath Mitra, Sushant Ciliveri, Jose D Avila, William Dernell, Stuart B Goodman and Susmita Bose.
These implants are fabricated via multi-material 3D Printing or additive manufacturing to impart continued bacterial resistance while enabling early bone formation through enhanced biocompatibility. Image Credit: Amit Bandyopadhyay, Indranath Mitra, Sushant Ciliveri, Jose D Avila, William Dernell, Stuart B Goodman and Susmita Bose.
Prof. Amit Bandyopadhyay’s team published an easy but incredibly efficient solution to this issue in the International Journal of Extreme Manufacturing. They recommended adding 3-weight percent copper (Cu) to titanium (Ti) implants by multi-material additive fabrication. This improves biocompatibility by adding tantalum (Ta), which promotes early-stage osseointegration and naturally guards against bacterial infection.
A recent World Health Organization report stated the alarming fact that around 700 000 deaths occur annually due to antimicrobial resistance (AMR). If effective clinical remedies are not found, the number is projected to surge to as many as 10 million deaths per year by 2050—higher than 8.2 million deaths per year due to cancer, making AMR a substantial global economic burden.
Amit Bandyopadhyay, Study First and Corresponding Author and Boeing Distinguished Professorship, Washington State University
In addition, the death rate from prosthetic joint infection (PJI) is an astounding 87.3%, higher than the rates for lung and colorectal cancer and almost the same as for breast cancer (89%). It is obvious that PJI has developed into a serious clinical issue that has to be addressed right away.
Regretfully, current methods of resolving these problems are not always viable, which might result in recurrent infections after revision surgery. The issue is made more difficult by the mutual exclusion of high infection rates, revision surgeries, and the out-of-pocket expenses related to these treatments. Implants that are self-sufficient in preventing PJI and reducing the complexity of revision operations are, therefore, desperately needed.
Infection control and improved biocompatibility are two important issues that are still unfulfilled in today’s metallic material choices for these biomedical applications. The most widely used metallic biomaterial for load-bearing implants at the moment is Ti6Al4V.
Nevertheless, studies have also revealed that Ti6Al4V is not as biocompatible as pure Ti, which causes a delay in the healing process and affects patients with weak bones. Conversely, pure Ti exhibits outstanding biocompatibility, yet its mechanical characteristics make it unsuitable for applications requiring load-bearing.
Bandyopadhyay added, “So the question is whether we can design an alloy composition between pure Ti and Ti6Al4V with biocompatibility similar to pure Ti while mechanical strength is close to or similar to Ti6Al4V. Furthermore, can that composition be additively manufactured explicitly to design patient-specific load-bearing implants as an alternative to Ti6Al4V?”
Researchers have developed a variety of multifunctional materials, such as Ti-Cu alloys, to replace Ti6Al4V for higher biocompatibility. These materials have the potential to boost product value, lower healthcare costs, and raise implant success rates.
Nevertheless, most research has only assessed Ti implants made using powder metallurgy and alloyed with ≥ 5% Cu to provide bacterial resistance. The scientific community is increasingly concerned about the implant materials’ high Cu concentration.
Moreover, these investigations frequently neglect the possible cytotoxicity linked to increased Cu levels or neglect to assess methods for fully enhancing the implants’ early-stage osseointegration capacity.
“Adding Cu may alleviate the issue of bacterial colonization and improve implant biocompatibility, but we must also consider the potential impact of Cu on bone response when implanted in vivo. A promising solution should be a functional material that is universally applicable but simultaneously addresses various drawbacks contributing to poor biocompatibility. Therefore, we are dedicated to exploring the extent of alterations and establishing an alternative to mitigate any compromise in biocompatibility,” Bandyopadhyay further noted.
Using laser powder bed fusion (LPBF), Prof. Bandyopadhyay’s group created Ti3Al2V alloy by combining CpTi and Ti6Al4V powders in a 1:1 weight ratio. In addition, to improve biocompatibility and provide intrinsic bacterial resistance, 3 weight percent Cu (3Cu) and 10 weight percent Ta (10Ta) were added to the Ti3Al2V alloy.
They next examined the effectiveness of the additively manufactured implants against strains of Pseudomonas aeruginosa and Staphylococcus aureus bacteria for up to 48 hours. Their hard work paid off, as their created Ti3Al2V-10Ta-3Cu alloy showed a remarkable synergistic effect on improving both microbial resistance and in vivo biocompatibility.
It was noteworthy that it showed an antibacterial efficacy that was 80% greater than that of conventional CpTi and Ti6Al4V, indicating a major advancement toward more effective customized therapy for the upcoming generation of load-bearing metallic implants.
Bandyopadhyay further stated, “Our novel Ti3Al2V alloy exhibits excellent fatigue resistance, exceptional shear strength, and improved tribological and tribo-biocorrosion characteristics compared to its counterparts, CpTi and Ti6Al4V. This achievement is due to the deliberate reduction of the Vanadium (V) and Aluminum (Al) components from Ti6Al4V, as these elements do not add any significant biological properties to these alloys. Importantly, these improvements are realized without compromising any mechanical properties of these alloys.”
Prof. Bandyopadhyay concurs that before these materials are adopted for human use, more extensive, longer-term in vivo experiments with large animals at the device level are required. To achieve >99% efficiency in preventing bacterial colonization, he would also like to see ongoing efforts to advance compositional change.
He concluded, “But ultimately, we feel a material like Ti3Al2V-10Ta-3Cu could improve patients’ postoperative quality of life and reduce the number of revision surgeries that arise from multiple scenarios, including bacterial infection.”
Journal Reference:
Bandyopadhyay, A., et al. (2023) Additively manufactured Ti–Ta–Cu alloys for the next-generation load-bearing implants. International Journal of Extreme Manufacturing. doi:10.1088/2631-7990/ad07e7.